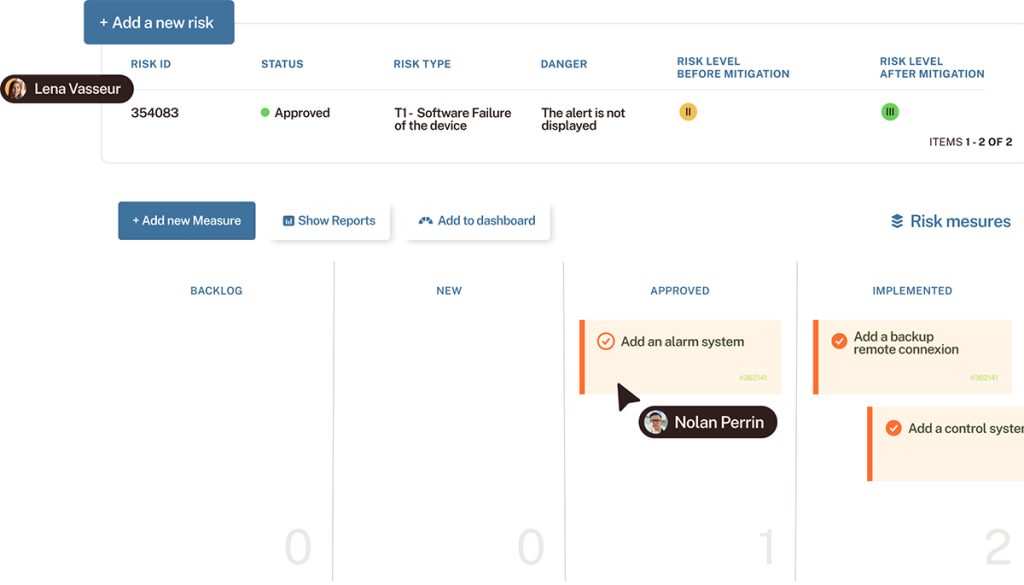
Ensure quality in software development
- Software Quality: understanding the different types of software testing
- Developing industry compliant software with an ALM tool
- How traceability hits compliance and quality software development
- Demo – Quality Assurance and Compliance with Tuleap Test Management
- Customer Success Story: How did STMicroelectronics set up a quality assurance plan to achieve ISO compliance?
Agility & Standards Compliance
- Agile and Compliant: both achievable and valuable
- Standards Compliance: do Auditors really approve Agile approaches?
- 7 tips to use Agile practices as a lever for Compliance
The standards of the Medical Device industry
- IEC 62304: What are the requirements for Medical Device Software?
- IEC 62304 standard for medical device: What do you risk if you don’t comply with it?
- ISO 13485: What makes a good Quality Management System (QMS) for Medical software?
- ISO 13485 – Why implement a Quality Management System (QMS) for medical device software?
- ISO 13485: How to implement a Quality Management System (QMS) with Tuleap?
- IEC 62304: how Tuleap ensures easier and cost-effective standard compliance for medical device software
- Customer Success Story: SleepInnov Technology achieves ISO 13485 & IEC 62304 Medical Device compliance
- Ready-to-use Tuleap Template for the medical industry
The standards of the Automotive industry
- ISO 26262: deploying an Application Lifecycle Management tool to make compliance easier
- Automotive functional safety and ISO 26262: relevance and deployment within the industry
- ISO 26262 – ASPICE: developing compliant and innovative automotive software with Tuleap