Healthcare and medical devices
Innovate faster, control risks, and simplify audits with Tuleap.
Available in EN/FR
Available in the cloud and on-premises
All-in-one plateform

#134009
US-002-001: System error detection

#134010
US-002-002: Automated error handling

Healthcare and medical devices: innovation in support of compliance
In a highly regulated MedTech environment, you need to innovate quickly while ensuring the compliance and quality of your medical devices.
Accelerate innovation without sacrificing quality
Speed to market is a competitive advantage, but it must never come at the expense of safety or compliance.
How does Tuleap address this?
Standards built in from day one
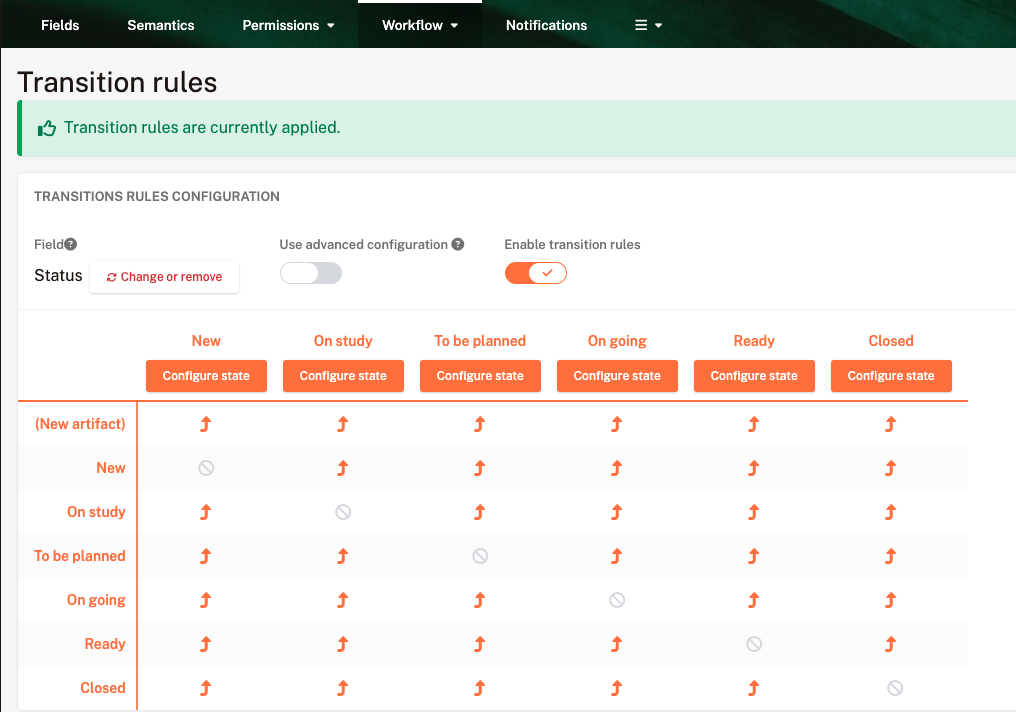
Use tailored workflows to meet regulatory requirements without sacrificing agility, and save time by automating risk management, requirements, and compliance evidence, for more reliable and cost-effective processes.
Templates tailored to your constraints
Configure your templates to match your standards (ISO 13485, etc.) and reuse them across all your projects.
“
Face audits with confidence
Regular audits require comprehensive, easily accessible evidence of compliance.
How does Tuleap address this?
Audit-ready compliance evidence
Automatically generate traceability reports and documentation for stress-free audits.
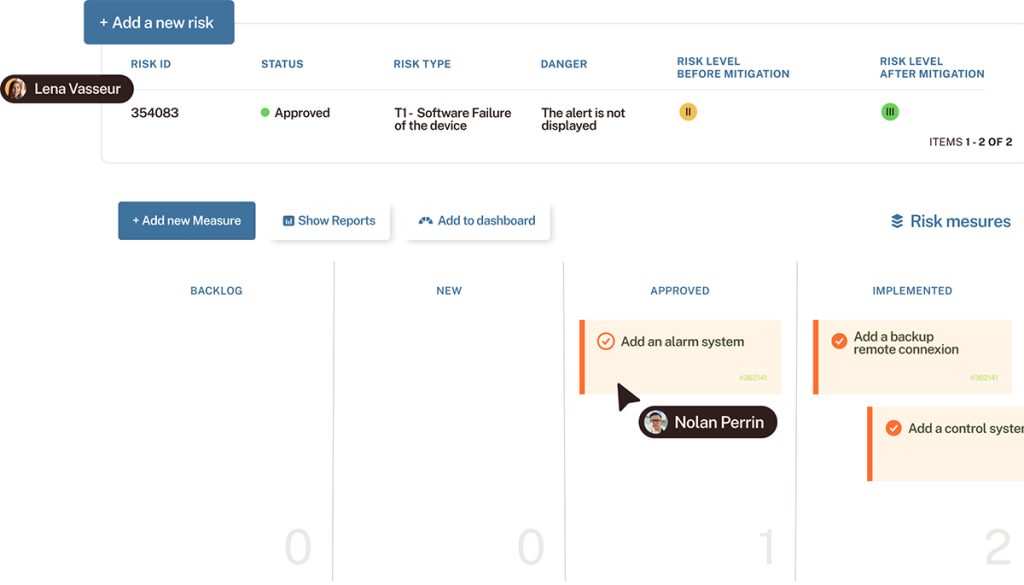
Proactive risk management
Identify, assess, and mitigate risks from the very start of the project, with custom workflows and automated alerts.
Always up-to-date documentation
Share, review, and archive documents securely, with granular access control and a complete audit trail.

“

“
Collaborate effectively across disciplines
The success of a MedTech project depends on close coordination between technical, regulatory, and business teams.
How does Tuleap support you?
Unified workspace
Work in a unified environment where developers, testers, and quality managers share the same tools and information.
Centralized information
Give each team (engineering, quality, safety) real-time access to the data it needs.
Collaborative workflows
Automate approvals and reviews to speed up decision-making.
Ensure the long-term sustainability of medical devices
Maintenance and evolution of medical devices must be anticipated from the design stage.
How does Tuleap address this?
Automated generation of compliance evidence
Document and track every change, from code to requirements, to ensure efficient maintenance and long-term compliance.
Adapt to evolving standards
Update your processes in real time, with flexible tools and automated alerts to stay compliant.
They trust us
















Our success stories
See how organizations in the medical sector structure their projects, improve traceability, and deliver faster with Tuleap.


- eBook, Success story
How Softway Medical achieves 300 people alignment and simplifies ISO 13485 compliance?
- eBook, Success story
How does Sleepinnov Technology ensure its compliance to ISO 13485 and IEC 62304 standards for medical device software with Tuleap?
Solution overview
Tuleap brings requirements, development, testing and documentation together in a single platform.
Designed to connect teams, secure processes and ensure continuous traceability in complex environments.

Support your agile transformation
Reduce resistance to change, improve collaboration and track progress across your agile practices with a platform built for continuous improvement.
Frequently asked questions

Is Tuleap suitable for small MedTech organizations?
Yes. Tuleap is scalable and fits both startups and large enterprises, with the same modular features and controlled costs.
Can workflows be customized to match our internal processes?
Absolutely. Tuleap lets you create custom workflows tailored to your working methods and regulatory requirements.
How does Tuleap make regulatory requirements management easier?
By automatically linking requirements, tests, and code, and generating audit-ready compliance evidence, Tuleap simplifies standards management.
Why Tuleap?
Tuleap helps organizations reduce project risks, improve software quality, and gain full control over their delivery cycles with a comprehensive and flexible ALM platform.
One single tool
The platform brings together project management, requirements tracking, testing, and collaboration. Teams reduce tool sprawl, keep workflows under control, and deliver more consistently.
Track every requirement without gaps
Tuleap links requirements, tasks, tests, and defects in a single workspace. Teams gain continuous visibility across the development lifecycle, making audits, compliance, and project decisions easier.

Adapt the tool to your organization
Tuleap integrates into a wide range of environments, on-premises or in the cloud, with full control over data. Workflows, trackers, and templates adapt to internal practices without adding governance complexity.
Improve software quality and delivery timelines
The platform gives teams a clear view of priorities, risks, and progress. Decisions are better informed, and deliveries gain reliability across multi-disciplinary environments.
Discover our latest posts
Articles and insights to help you understand your challenges and strengthen your practices.

- ALM - PLM
Tuleap has been mentioned twice by Gartner®: In the “Hype Cycle for (...)

In project management, finding the right information at the right time is (...)
- Switch from Jira
End of Jira Data Center: prepare for the future now! Atlassian has (...)