What is IEC 62304?
IEC 62304 is the international standard that defines the life cycle requirements for medical device software. It is a fundamental reference for medical device requirements engineering and for organizations developing software in medical devices or standalone medical device software (SaMD). The standard is recognized by regulatory authorities worldwide, including the FDA and the European Union, as an essential benchmark for medical device compliance software and medical device software engineering.
What are the IEC 62304 Safety Classifications?
The IEC 62304 standard introduces 3 software safety classes, from A (least critical) to C (most critical). The more crucial the classification, the more comprehensive the software lifecycle must be.
Software safety classes are defined by the severity of the consequences of a software failure:
- Class A: no injury or damage to health is possible
- Class B: non-serious injury is possible
- Class C: death or serious injury is possible
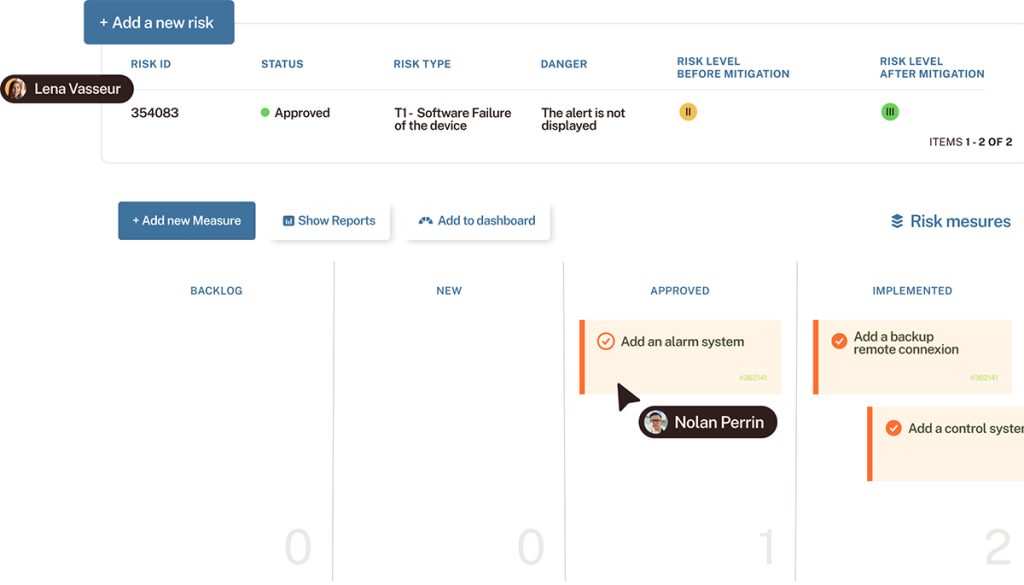
The IEC 62304 standard requires a full software lifecycle framework including risk management, development, maintenance, configuration, and issue resolution — starting from Class A.
The impact of security classification on software engineering
Medical device regulations impose greater demands on design and development when potential failures could have significant consequences. Safety classification directly impacts the level of documentation, traceability, and risk management required throughout the lifecycle.
Read on
Want to go further on MedTech compliance challenges?
Download the MedTech White Paper →