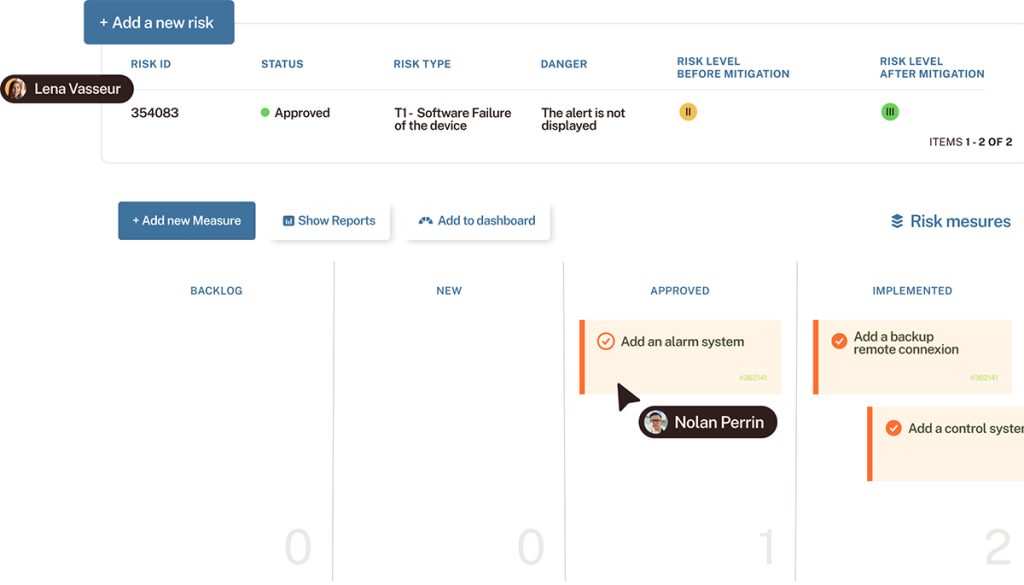
Sleepinnov Technology is a high-tech company that develops medical devices for the diagnosis and monitoring of patients with respiratory disorders (sleep apnea, respiratory failure, etc.). Its products are designed for healthcare professionals and are marketed in France and internationally. Thanks to Tuleap, Sleepinnov has implemented processes that ensure traceability and consistency at every stage of the development lifecycle of its medical devices.

Their results with Tuleap
4x more efficient project management
Standardized processes provide greater clarity and consistency across all stages of software development.
2x improvement in deliverable quality management
“Today, thanks to Tuleap, bugs are identified and resolved much faster.”
100% traceability objective achieved
End-to-end traceability strengthens confidence, safety, and efficiency during ISO 13485 and IEC 62304 compliance audits.

Tuleap has brought greater security, efficiency, and confidence to our ISO 13485 audit processes
for medical devices.
Testimony of Nicolas GAIFFE, Software Development Manager at Sleepinnov Technology
Nicolas Gaiffe is responsible for software development in the medical domain at Sleepinnov Technology.
In this customer case study, he explains why and how Sleepinnov relies on Tuleap.
Fill out the form to get access to the full customer case study.