Common use cases of Tuleap Software Medical Device template
Tuleap implementation helps you solve the following business challenges:
- How to improve your medical device software management across teams?
- How to implement an efficient quality management system to meet the medical device industry standards?
- How to ensure the full traceability throughout the lifecycle of your MD software products?
- How to ensure the delivery of validated, high-quality products?
- How to prepare and demonstrate ISO 13485 and IEC 62304 compliance with less effort?
Tuleap Template for Software Medical Device
Tuleap ready-to-use template for medical device software helps you define and implement an effective process to optimize the management of your MD projects as well as to provide proof of the quality of your delivered products. Benefit from a preset, integrated environnement, providing all the necessary workspaces and tools to enhance each step of your software project management.
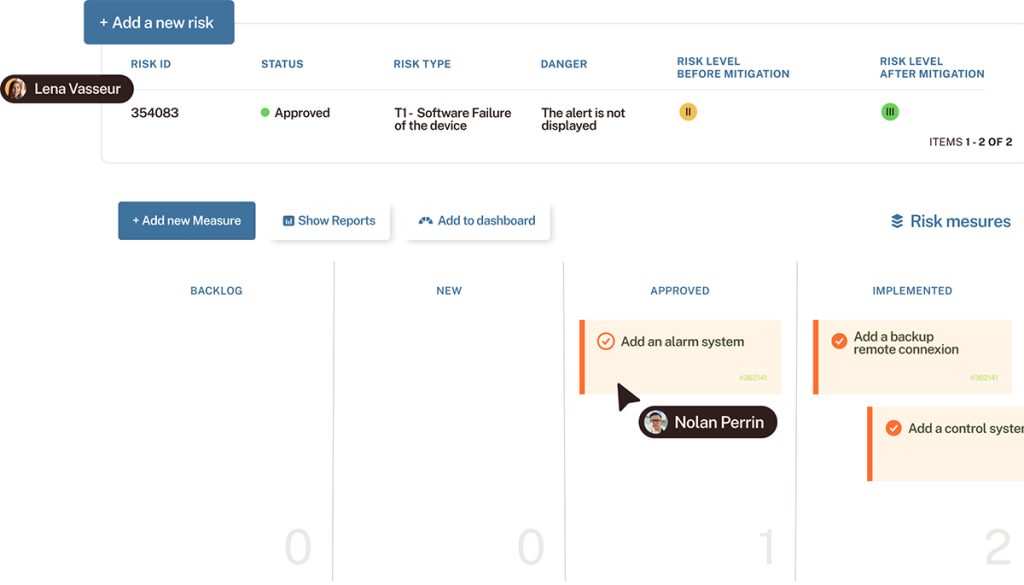
- Manage all medical work items as requirements, risks and tasks,
- Visualize your product roadmap in real-time,
- Organize the product backlog, define the milestones and plan features development,
- Customize Kanban boards and workflows to better follow projects progress,
- Create and launch test campaigns to validate both features and requirements compliance,
- Generate a detailed technical document to report the validation campaigns’ results thanks to Tuleap DocGen™ plugin,
- Get a traceability matrix to prove the quality of your medical device software for audit purposes.
At any moment, you can change the settings of your configuration.
Examples of usage




See this template live
Developing medical device software compliant with ISO 13485 and IEC 62304
It is time for action now. Let’s provide teams with the right tools to define and optimize the management of medical device (MD) projects and provide proof of the quality of your software for audit compliance purposes.