Ensuring compliance with standards like IEC 62304, ISO 13485, or MDR is not optional.
Learn how to structure your development projects to guarantee full traceability, without slowing down innovation.
Are you looking to simplify compliance management for your medical devices and software?
We’ll show you how centralizing requirements, automating regulatory processes, and seamlessly integrating them into your development cycle can help you meet regulatory standards, without overloading your teams with excessive administrative tasks.
What you will learn:
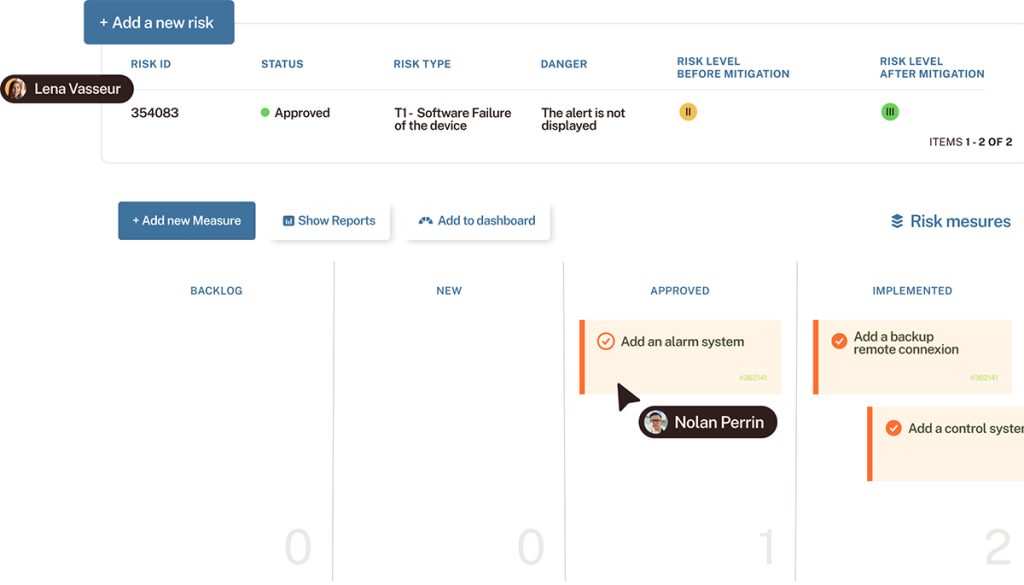
– How to structure your requirements, tests, defects, and risks to ensure end-to-end traceability
– How to meet IEC 62304 and ISO 13485 requirements with a single, integrated tool
– How to align R&D, Quality, and Regulatory teams without creating silos
– How to prepare for audits stress-free (and without relying on spreadsheets)
– A concrete example of a quality process powered by Tuleap
– Real-world feedback: Insights from MedTech professionals
🧑⚕️ Who should attend?
This webinar is for you if you are:
– A Quality or Compliance Manager in the MedTech industry
– A Software or Systems Project Manager
– An R&D, Validation, or Regulatory Affairs Engineer
– A supplier of embedded software or Software as a Medical Device (SaMD)